Efficacy of Pfizer-BioNTech COVID-19 vaccine on adolescents
Source:R/data-biontech_adolescents.R
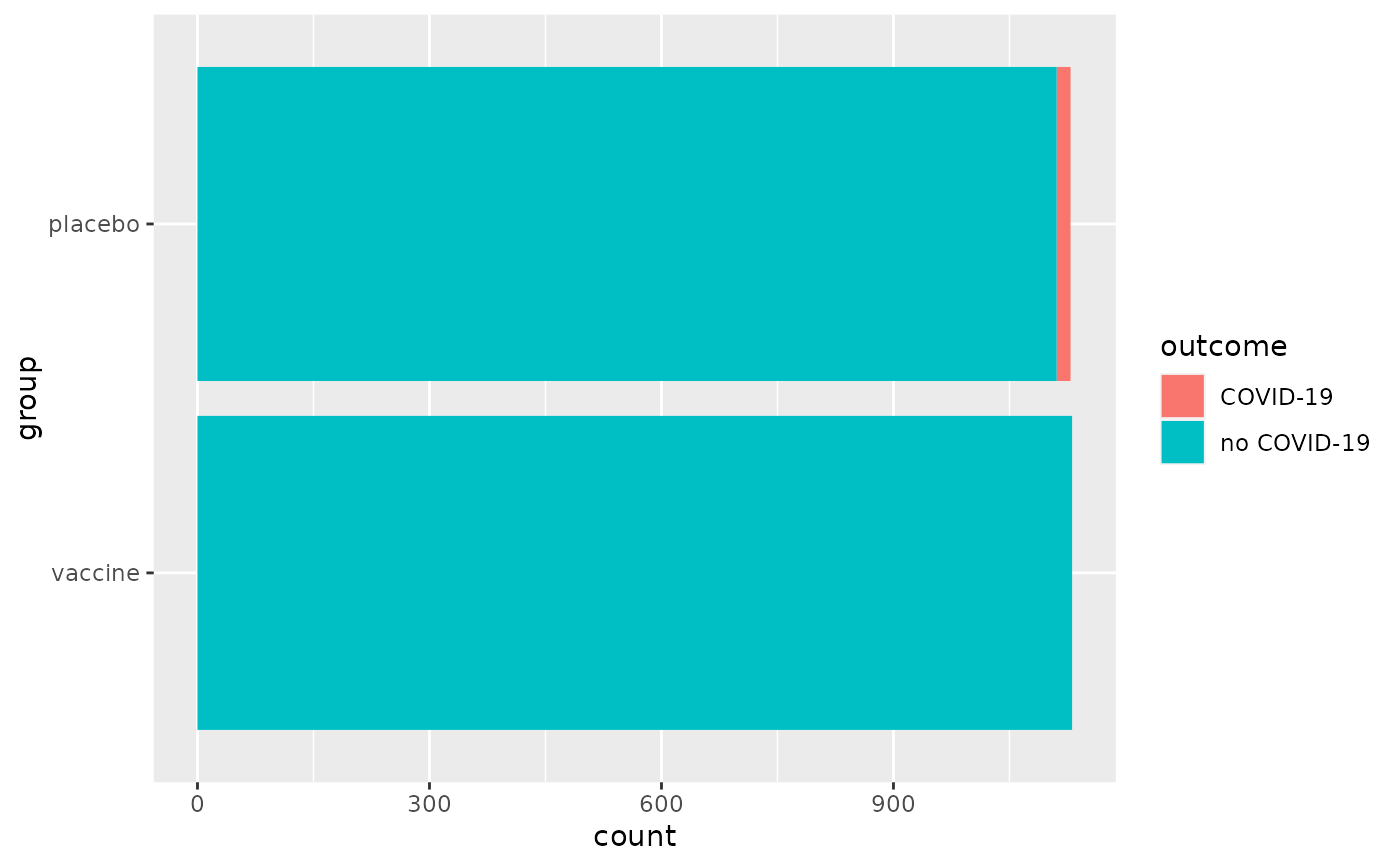
biontech_adolescents.RdOn March 31, 2021, Pfizer and BioNTech announced that "in a Phase 3 trial in adolescents 12 to 15 years of age with or without prior evidence of SARS-CoV-2 infection, the Pfizer-BioNTech COVID-19 vaccine BNT162b2 demonstrated 100% efficacy and robust antibody responses, exceeding those recorded earlier in vaccinated participants aged 16 to 25 years old, and was well tolerated." These results are from a Phase 3 trial in 2,260 adolescents 12 to 15 years of age in the United States. In the trial, 18 cases of COVID-19 were observed in the placebo group (n = 1,129) versus none in the vaccinated group (n = 1,131).
Format
A data frame with 2260 observations on the following 2 variables.
- group
Study group:
vaccine(Pfizer-BioNTech COVID-19 vaccine administered) orplacebo.- outcome
Study outcome:
COVID-19orno COVID-19.
Source
"Pfizer-Biontech Announce Positive Topline Results Of Pivotal Covid-19 Vaccine Study In Adolescents". March 21, 2021. (Retrieved April 25, 2021.)